Refractory failure is the silent killer of industrial profitability, often striking when furnace loads are highest. Cracks in the lining, molten slag penetration, and catastrophic spalling can halt production instantly. These shutdowns do not just cost material; they bleed operational budgets through unplanned downtime and maintenance labor. Here is the deal: you cannot afford to guess when selecting lining materials for high-temperature environments.
The solution lies in selecting the correct phase-stabilized zirconia. Whether your operation requires the thermal shock resistance of Magnesia-Partially Stabilized Zirconia (MgO-PSZ) or the chemical inertness of Yttria-Stabilized Zirconia (YSZ) determines your facility’s efficiency. We provide the expertise to navigate these complex chemical choices, ensuring your linings last longer and your furnaces run hotter. Zirconia Stabilized Refractories are not just commodities; they are engineered solutions for extreme environments. By understanding the distinct advantages of each stabilizer, you can transform your refractory strategy from a reactive maintenance burden into a proactive competitive advantage.
Why Must Zirconia Be Stabilized?
Zirconia (ZrO₂) requires stabilization because pure zirconia undergoes disruptive phase transformations during cooling that cause structural failure. As the material cools from high fabrication temperatures, it shifts from a tetragonal to a monoclinic crystal structure. You might be wondering: why is this transformation so dangerous for industrial ceramics?
The Phase Transformation Problem
This phase shift is accompanied by a volume expansion of approximately 3% to 5%. In a rigid ceramic body, an internal expansion of this magnitude creates massive internal stress. Here is the kicker: without stabilization, these stresses exceed the material’s strength, causing the refractory to crack or crumble into dust upon cooling. This makes pure, unmodified zirconia useless for structural applications in high-heat environments. The volume expansion is powerful enough to shatter the ceramic bonds, turning what should be a solid brick into a pile of useless powder.
The Role of Chemical Stabilizers
Engineers introduce stabilizing oxides like Magnesium Oxide (MgO), Calcium Oxide (CaO), or Yttrium Oxide (Y₂O₃) to solve this. These dopants enter the crystal lattice and lock the structure in its high-temperature cubic or tetragonal phase. What does this mean for you? It means the material retains its shape and strength from room temperature up to 2000°C or higher, preventing that catastrophic volume expansion. This chemical doping effectively “freezes” the high-temperature crystal structure, allowing the material to function as a reliable solid.
Comparing Pure and Stabilized States
Stabilization is not optional; it is the fundamental chemical modification that transforms zirconia from a liability into a high-performance refractory asset. Without it, the material is mechanically unstable.
| Property | Pure Zirconia (Monoclinic) | Stabilized Zirconia (Cubic/Tetragonal) | |
|---|---|---|---|
| Crystal Structure | Unstable during cooling | Locked/Stable | |
| Volume Change | 3-5% Expansion (Cracks) | Negligible (<1%) | |
| Thermal Limit | ~1170°C (Transformation point) | >2400°C | |
| Industrial Viability | None (Dusting) | High (Structural Integrity) |
How Are Zirconia Stabilized Refractories Made?
The manufacturing process involves precise powder metallurgy techniques where high-purity zirconium dioxide is mixed with specific stabilizing oxides. This mixture is not merely blended; it undergoes rigorous processing to ensure the stabilizer is uniformly distributed within the crystal lattice. So, how does this happen?
Raw Material Selection and Mixing
Production begins with selecting high-purity monoclinic zirconia powders and the chosen stabilizer (MgO or Y₂O₃). Here is the secret: the particle size distribution must be strictly controlled. Manufacturers wet-mill these powders to create a homogeneous slurry, ensuring that the stabilizing ions can physically migrate into the zirconia structure during heating. Binders are added to help the powder hold its shape during the initial pressing phase, creating a “green body” that is fragile but formed.
The Sintering Process
After the powder is pressed into green bodies (unfired shapes), they enter the kiln for sintering. Ready for the hard part? The material must be fired at temperatures ranging from 1500°C to 1750°C. During this soak time, the material densifies, and the stabilizing oxides diffuse into the zirconia lattice. For MgO-PSZ, a specific cooling cycle is required to precipitate the “toughening” tetragonal phase within the cubic matrix, a critical step that defines its mechanical properties. This controlled cooling, or “aging,” is what gives the material its unique ability to resist cracking.
Key Manufacturing Parameters
The performance of the final refractory is dictated not just by chemistry, but by the precise control of the sintering curve and cooling rate during manufacturing.
| Manufacturing Step | MgO-PSZ Requirement | YSZ Requirement | |
|---|---|---|---|
| Stabilizer Content | ~3.0 – 3.5 wt% (Mg) | ~5.0 – 8.0 wt% (Y) | |
| Sintering Temp | High (~1700°C) | Moderate (~1550°C) | |
| Cooling Profile | Controlled aging required | Standard cooling | |
| Microstructure | Precipitated matrix | Uniform grain structure |
How Does Stabilizer Type Impact Refractory Performance?
The specific oxide used to stabilize the zirconia dictates the material’s thermal shock resistance, corrosion profile, and maximum operating temperature. While all stabilizers prevent dusting, they impart vastly different physical characteristics to the final brick or nozzle. Let’s look at the details.
Chemical Interactions with Slag
The choice of stabilizer fundamentally changes how the refractory reacts with your process environment. Here is a fact: Magnesia (MgO) is basic, making MgO-PSZ suitable for basic slags but vulnerable to acidic environments. Yttria (Y₂O₃), conversely, is more chemically inert and stable. If you put the wrong stabilizer into an aggressive slag system, chemical leaching will dissolve the bonds holding the grains together, causing rapid erosion. The stabilizer itself can become the weak link if mismatched with the process chemistry.
Thermal Expansion Rates
Different dopants alter the coefficient of thermal expansion (CTE). Why does this matter? If the refractory expands at a different rate than the surrounding steel shell or adjacent masonry, mechanical stresses build up. MgO-stabilized materials generally have lower expansion rates compared to CaO-stabilized variants. This lower expansion reduces the mechanical shearing forces during heat-up, contributing to a longer service life in cyclic applications. Understanding these thermal dynamics is critical for preventing structural failure of the furnace lining itself.
Stabilizer Performance Matrix
You cannot swap stabilizers arbitrarily; you must match the stabilizer’s chemical nature (acid/base) and physical properties to your specific furnace conditions.
| Feature | MgO-Stabilized | CaO-Stabilized | Y₂O₃-Stabilized | |
|---|---|---|---|---|
| Chemical Nature | Basic | Basic | Amphoteric/Inert | |
| Thermal Expansion | Moderate | High | Moderate | |
| Slag Resistance | Good (Basic Slags) | Good (Glass) | Excellent (Universal) | |
| Cost Profile | Moderate | Low | High |

What Makes MgO-PSZ a Tough Choice for Refractories?
MgO-PSZ is engineered specifically to withstand mechanical abuse and rapid temperature swings that would shatter other ceramics. It is widely considered the “toughest” of the zirconia family because of its unique microstructural design. How does it do this?
Mechanism of Transformation Toughening
The material is “Partially Stabilized,” meaning it contains a cubic matrix with small precipitates of metastable tetragonal zirconia. This is where it gets interesting: When a crack tries to move through the material, the stress energy triggers these precipitates to expand and transform. This expansion squeezes the crack shut, effectively stopping it from propagating. It is an active defense mechanism within the ceramic itself, absorbing the energy that would otherwise cause a catastrophic failure.
Ideal Environments for MgO-PSZ
Because of this toughness, MgO-PSZ is the standard for cyclic applications. Picture this: A steel ladle nozzle that goes from room temperature to 1600°C in seconds. MgO-PSZ absorbs this shock without spalling. It withstands the erosion of flowing molten steel and the mechanical impact of solidified slag removal. However, care must be taken in vacuum environments, as MgO has a high vapor pressure and can volatilize at extreme temperatures.
MgO-PSZ Critical Statistics
Choose MgO-PSZ for intermittent processes where thermal cycling is frequent and mechanical toughness is the primary requirement preventing failure.
| Property | Value / Description | Impact on Operation | |
|---|---|---|---|
| Fracture Toughness | Very High | Resists cracking during impact | |
| Thermal Shock | Excellent | Survives rapid heating/cooling | |
| Vapor Pressure | High at >1500°C | Avoid high-vacuum use | |
| Primary Use | Steel/Foundry | Ladle nozzles, slide gates |

How Does CaO-PSZ Provide Stability During High-Temperature Operation?
Calcium Oxide-Partially Stabilized Zirconia (CaO-PSZ) is the workhorse for long-duration, steady-state high-temperature campaigns. While it may lack the extreme impact toughness of MgO, it makes up for it with structural reliability over time. What is the difference?
Long-Soak Stability
CaO-PSZ is exceptionally stable when held at high temperatures for weeks or months. Here is the deal: Unlike MgO, which can migrate or volatilize during long heat soaks, the calcium ion remains locked in the lattice. This makes it the preferred material for continuous kiln linings or glass tank flux blocks where the furnace runs 24/7 without cooling down. It resists “creep” (deformation under load) better than many alternatives, ensuring the furnace geometry remains constant throughout the campaign.
Resistance to Alkalis
In the glass industry, refractories are constantly attacked by alkaline vapors and molten glass. But here’s the kicker: CaO is naturally resistant to these alkaline environments. It does not react as aggressively with the calcium and sodium found in glass batches. This chemical compatibility prevents the “rat-holing” and erosion often seen at the melt line in glass production furnaces, extending the life of the tank blocks significantly.
CaO-PSZ Performance Data
If your furnace runs continuously at temperatures above 1600°C, especially in glass manufacturing, CaO-PSZ offers the best structural stability.
| Characteristic | CaO-PSZ Performance | Advantage | |
|---|---|---|---|
| Creep Resistance | High | Maintains shape under load | |
| Alkali Resistance | Excellent | Resists glass corrosion | |
| Stability Temp | >1600°C | Suitable for long campaigns | |
| Destabilization | Low | Less phase degradation over time |
What Sets YSZ Apart as the Superior Refractory Performer?
Yttria-Stabilized Zirconia (YSZ) is considered the premium tier of the zirconia family. It offers a combination of properties that neither Mg nor Ca can match, primarily due to the unique properties of the yttrium ion. Why is it superior?
Unmatched Chemical Inertness
Yttrium oxide is chemically incredibly stable. What’s the real story? YSZ does not react with most acidic or basic slags, nor does it react with sensitive electronic grade powders being fired in a kiln. While MgO might leach into a melt, YSZ remains practically invisible chemically. This purity makes it essential for firing high-tech ceramics (like piezoelectric parts) or melting reactive alloys like titanium, where contamination must be zero. The lack of reactivity means the lining stays intact longer and the product stays purer.
Thermal Efficiency and Insulation
YSZ has one of the lowest thermal conductivities of any dense ceramic. Bottom line? It acts as a super-insulator. In a refractory lining, a layer of YSZ prevents heat from escaping the furnace, driving up energy efficiency. This property is also why YSZ is used as a thermal barrier coating in jet engines—it protects the metal blades from melting. It combines this insulation with the highest phase stability, meaning it rarely suffers from the degradation that affects other types.
YSZ Performance Metrics
YSZ is the choice when failure is not an option, chemical purity is mandatory, or maximum thermal insulation is required.
| Feature | YSZ Capability | Operational Benefit | |
|---|---|---|---|
| Reactivity | Extremely Low | Zero product contamination | |
| Thermal Conductivity | Ultra-Low (<2.5 W/mK) | Saves energy / Protects shell | |
| Phase Stability | Highest | Longest reliable service life | |
| Melting Point | ~2700°C | Withstands extreme heat |
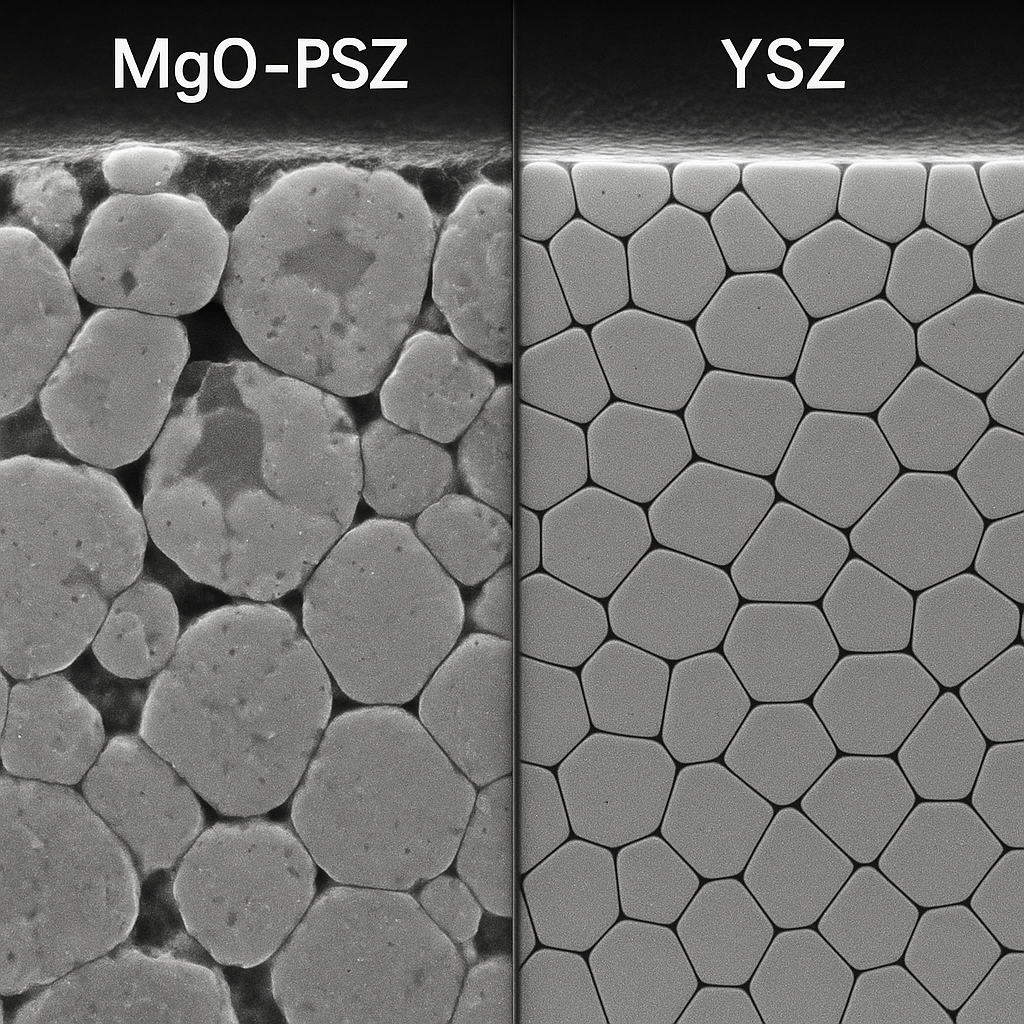
Where Are Zirconia Stabilized Refractories Used?
These materials are not interchangeable; specific industries rely on specific stabilizers to maintain production. Let’s break it down. The application usually dictates the stabilizer choice based on the dominant failure mode—whether it is thermal shock, chemical attack, or long-term creep.
Steel and Foundry Applications
In the steel industry, you will find MgO-PSZ dominating. Continuous casting nozzles, slide gate plates, and break rings rely on MgO-PSZ. The molten steel is aggressive, and the process involves violent temperature shocks. MgO-PSZ survives the start-up of a cast where temperature jumps 1500 degrees instantly. It also resists the erosion of tons of steel flowing through a small orifice, ensuring precise flow control for the duration of the cast.
Glass and Electronics Manufacturing
The glass industry leans toward CaO-PSZ and fused cast AZS (Alumina-Zirconia-Silica). Here is why: They need resistance to alkaline corrosion over campaigns lasting years. Conversely, the electronics industry uses YSZ setter plates. When sintering sensitive capacitors or piezoelectric ceramics, the furniture (refractory) cannot react with the product. YSZ provides a non-reactive surface that ensures the electronic components remain pure and functionally compliant.
Application Matrix by Industry
Match the refractory to the industry’s specific stress factors: MgO for Steel (Shock), CaO for Glass (Chemical/Time), YSZ for Electronics (Purity).
| Industry | Preferred Material | Primary Component | Reason | |
|---|---|---|---|---|
| Steel | MgO-PSZ | Metering Nozzles | Thermal Shock | |
| Glass | CaO-PSZ | Tank Blocks | Alkaline Resistance | |
| Aerospace | YSZ | Barrier Coatings | Thermal Insulation | |
| Electronics | YSZ | Kiln Furniture | Chemical Purity |

Are Zirconia Stabilized Refractories Cost-Effective?
Purchasing managers often balk at the price of zirconia refractories compared to alumina or magnesia-carbon bricks. Zirconia is significantly more expensive. But consider this: the cost of the brick is irrelevant compared to the cost of the shutdown.
Initial Investment Analysis
YSZ is the most expensive, followed by MgO-PSZ, with CaO-PSZ often being the most economical of the high-performance group. The reality is: The raw material cost of Yttria is high, and the processing of zirconia requires high temperatures, driving up energy costs during manufacturing. A YSZ lining might cost 3x to 5x more than a high-alumina equivalent. However, this upfront cost buys you superior material properties that cheaper alternatives simply cannot possess.
Long-Term ROI
However, if a standard nozzle lasts 4 hours and a zirconia nozzle lasts 20 hours, the ROI is massive. Here is the math: You reduce the frequency of sequence changes, you reduce the risk of breakouts (which can cost millions), and you improve steel cleanliness. In high-value manufacturing, such as aerospace casting or continuous steel casting, the reliability of zirconia pays for itself in a single shift. The reduction in labor costs for maintenance and the increase in throughput far outweigh the initial material expense.
Cost vs. Lifespan Analysis
Do not buy based on price per ton. Buy based on cost per cast or cost per firing cycle. Zirconia is an investment in continuity.
| Material | Relative Cost | Lifespan Factor | Best Economic Use Case | |
|---|---|---|---|---|
| Alumina | $ | 1x | Low temp / Non-critical | |
| MgO-PSZ | $$$ | 5x – 10x | High wear / High shock | |
| YSZ | $$$$$ | 15x+ | Critical / Zero-fail zones | |
| ROI | N/A | High | Maximum |

How Can Professionals Quickly Select the Right Refractory?
Selection paralysis can happen when faced with technical data sheets. Let’s simplify it. You need to look at your primary failure mode. Is your current lining cracking from heat? Is it dissolving? Or is it contaminating your product?
Identifying Key Constraints
If your furnace cycles on and off daily, or if you are pouring metal intermittently, thermal shock is your enemy. So, what do you do? You select MgO-PSZ immediately. If your furnace runs continuously for 2 years making window glass, thermal shock does not matter, but chemical corrosion does. You select CaO-PSZ or Zircon. If you are sintering medical implants, purity is everything. You must pay the premium for YSZ. Using this diagnostic approach removes the guesswork.
Making the Final Call
Consult the chart below. It turns out: most decisions fall into three distinct buckets based on shock, corrosion, and purity. By categorizing your operational stresses, the material choice becomes obvious. Do not force a material into an application it wasn’t designed for; respect the chemistry.
Quick Selection Guide
Diagnose the failure mode of the previous lining to select the correct zirconia stabilizer for the next lining.
| Primary Constraint | Secondary Constraint | Recommended Material | |
|---|---|---|---|
| Thermal Cycling | Mechanical Impact | MgO-PSZ | |
| Chemical Attack | Continuous Heat | CaO-PSZ / YSZ | |
| Product Purity | High Value Parts | YSZ | |
| Budget | Static Temp | CaO-PSZ |

Why Is the Industry Shifting Towards YSZ?
Despite the cost, market trends show a clear shift toward Yttria-Stabilized Zirconia in high-end applications. You might ask: why spend the extra money? The answer lies in the increasing demands for efficiency and material performance limits.
Demand for Efficiency and Purity
Modern industrial processes are running hotter and faster to improve margins. Here is the deal: Traditional refractories cannot handle the new operating temperatures of advanced alloy production or the cleanliness requirements of modern steel. YSZ allows operators to push furnaces to 1800°C+ without risking a liner collapse. Furthermore, in the green energy sector (Solid Oxide Fuel Cells), YSZ is not just a refractory; it is an electrolyte, driving huge demand. The push for cleaner metals and higher efficiency engines necessitates materials that do not degrade.
The Reliability Factor
In automated 24/7 production lines, predictability is more valuable than low component cost. What does this mean? A YSZ component degrades at a predictable, slow rate. It rarely fails catastrophically without warning. This predictability allows maintenance managers to schedule downtime precisely, rather than reacting to emergency breakouts. The cost of an unscheduled stop in a modern automated plant dwarfs the cost of the premium refractory.
Market Growth Trends
The industry is moving to YSZ because the cost of failure has become higher than the cost of the material.
| Driver | Trend Description | Impact on Refractories | |
|---|---|---|---|
| Process Temp | Increasing globally | Need higher melting points (YSZ) | |
| Automation | 24/7 Operations | Need predictable wear patterns | |
| Quality | Clean Steel / Pure Glass | Need inert materials (No MgO leaching) | |
| Growth | +4% CAGR for YSZ | Shift from commodity to premium |
Conclusion
Refractory selection is rarely black and white, but the distinction between MgO-PSZ and YSZ is clear. MgO-PSZ is the champion of toughness, the rugged choice for the violent thermal world of steelmaking and foundries. YSZ is the master of stability, offering unmatched chemical inertness and longevity for critical, high-value applications where failure is not an option.
By choosing the right stabilizer, you eliminate the twin threats of thermal shock cracking and chemical corrosion. You transform your furnace lining from a consumable expense into a reliable production asset. Partner with Global Industry to analyze your specific operational parameters. We can help you transition to the zirconia grade that delivers the highest return on investment. The future of high-temperature processing is efficient, clean, and durable. Ensure your refractories are ready for it.
FAQ Section
Q1: What does stabilizing zirconia entail?Stabilizing zirconia involves adding oxides (MgO, CaO, Y₂O₃) to the material during manufacturing. This locks the crystal structure in a high-temperature phase (tetragonal/cubic), preventing the material from expanding and cracking when it cools down.
Q2: How does YSZ compare to MgO-PSZ and CaO-PSZ in terms of cost?YSZ is generally the most expensive due to the high cost of yttrium raw materials and processing. However, it often provides the best Return on Investment (ROI) for critical applications because it lasts significantly longer and reduces downtime.
Q3: In what environments do MgO-PSZ refractories thrive?MgO-PSZ thrives in environments with extreme thermal cycling and mechanical abuse, such as steel ladles and foundry casting nozzles. It is designed to resist cracking when temperatures change rapidly.
Q4: Why is chemical resistance significant in refractory applications?Chemical resistance prevents the refractory from reacting with the slag or the product. If a refractory has poor chemical resistance, it will dissolve into the molten metal or glass, destroying the lining and contaminating the final product.
Q5: Which refractory type should businesses choose for fluctuating high temperatures?For fluctuating temperatures involving mechanical shock, MgO-PSZ is best. However, for fluctuating temperatures requiring chemical stability and insulation without mechanical impact, YSZ offers superior phase stability and reliability.