Zirconia powder plays a crucial role in the development of medical implants due to its unique properties. However, many decision-makers grapple with the question: what specific technical requirements must zirconia powder meet for effective medical application? Addressing this concern is essential for those seeking reliable and durable implant materials that can enhance patient outcomes. This article offers a detailed exploration of critical standards, helping decision-makers in the medical field understand the necessary attributes of zirconia powder. We bring you insights backed by industry expertise, facilitating informed choices for your next project.

This image illustrates zirconia powder being utilized in the production of medical implants, showcasing its significance in healthcare.
What Are the Key Technical Requirements for Zirconia Powder?
Understanding the technical requirements for zirconia powder is vital for anyone involved in the selection of materials for medical implants. Zirconia, or zirconium dioxide (ZrO2), is distinguished by its exceptional properties, including high strength, durability, and biocompatibility, which make it a material of choice in various medical applications.
The first requirement is biocompatibility. Biocompatibility refers to how well a material interacts with the body, ensuring that it does not provoke an adverse reaction. For zirconia powder, passing rigorous biocompatibility tests is paramount for safe use in medical applications. This includes evaluations under ISO 10993 standards, which assess a material’s effect on living tissue.
Mechanical stability is another essential requirement. Zirconia must withstand various stresses without degrading, which is particularly crucial for load-bearing applications like dental or orthopedic implants. The material’s high flexural strength makes it resistant to fractures and other mechanical failures over time, ensuring that implants can support bodily functions without compromising structural integrity.
Lastly, wear resistance plays a crucial role in the performance of zirconia implants. Implants are subjected to continuous movement and loading, which necessitates that zirconia maintains its properties despite wear over time. A low wear rate is essential to prevent the release of particles into the body, which can lead to inflammation or other complications.
To summarize, the three pillars of zirconia’s technical requirements are biocompatibility, mechanical stability, and wear resistance. Prioritizing these attributes not only aids in regulatory compliance but also significantly enhances patient safety and outcomes, making zirconia an invaluable material in the medical field. Understanding these key requirements helps decision-makers choose the right materials, ultimately benefiting patient care.
| Technical Requirement | Importance | Key Parameter |
|---|---|---|
| Biocompatibility | Prevents adverse body reactions | ISO 10993 standards |
| Mechanical Stability | Ensures long-term performance | Flexural strength > 1000 MPa |
| Wear Resistance | Maintains integrity over time | Wear rate < 0.5 mm/year |
Why Is Biocompatibility Critical for Medical Implants?
Biocompatibility is a fundamental requirement for materials used in medical implants. It refers to a material’s ability to interact with the human body without causing any adverse effects. Impacts on patient safety are significant, as a lack of biocompatibility can lead to complications such as inflammation or rejection of the implant.
Regulatory standards mandate that materials used for implants pass biocompatibility tests. For instance, the ISO 10993 series provides guidelines for evaluating the biological effects of medical devices. Meeting these standards is essential for manufacturers looking to ensure that their products are safe for patients.
Furthermore, ensuring biocompatibility can directly influence patient outcomes. Successful integration of the implant into the body can lead to quicker recovery times and reduced post-operative complications. Ultimately, investing in high-quality zirconia powders that meet biocompatibility standards results in better patient satisfaction and trust in medical devices.
| Biocompatibility Tests | Purpose | Outcome |
|---|---|---|
| Cytotoxicity assessment | Evaluates cell viability in contact with material | No cell death |
| Sensitization test | Determines likelihood of immune response | Negative result preferred |
| Implantation studies | Assesses tissue response in living organisms | Good tissue integration |
How Does Mechanical Stability Affect Implant Longevity?
Mechanical stability is essential for the longevity of medical implants. It dictates how well the implant can endure the physical stresses placed upon it during normal body movements. Zirconia must exhibit exceptional mechanical properties to ensure it can perform as intended over time.
When evaluating mechanical stability, one of the key properties is flexural strength, which indicates the maximum stress a material can withstand while being bent. Zirconia typically has a flexural strength significantly higher than that of many other materials, making it suitable for high-load applications like hip or knee implants.
Fracture toughness is another crucial parameter to consider. It reflects the material’s ability to resist crack propagation. A high fracture toughness in zirconia contributes to the safety and longevity of the implant, minimizing the risk of failure.
For medical professionals and manufacturers alike, understanding these principles of mechanical stability helps foster confidence in zirconia as a material choice. The right evaluations can lead to enhancements in design and better performance outcomes.
| Mechanical Property | Description | Standard Value |
|---|---|---|
| Flexural Strength | Resistance to bending forces | > 1000 MPa |
| Fracture Toughness | Ability to resist crack growth | 4-6 MPa·m½ |
| Compressive Strength | Ability to withstand axial loading | > 2000 MPa |
What Role Does Wear Resistance Play in Zirconia Applications?
Wear resistance is a critical characteristic for materials used in implants. Due to the continuous motion and loading that implants endure, it is vital for zirconia to resist wear to ensure long-lasting performance.
Zirconia is exceptionally tough, which contributes to its excellent wear resistance. Its structure allows it to withstand friction and degradation when in contact with other surfaces in the body, such as cartilage and bones. This is particularly important in applications like dental implants, where zirconia must endure repeated chewing forces over time.
The rate of wear must also be carefully evaluated. Typically, zirconia has a wear rate lower than 0.5 millimeters per year, ensuring that it maintains its structural integrity and functionality. To emphasize, advancements in zirconia technology continue to enhance wear characteristics, yielding increasingly reliable materials for the medical field.
Thus, focus on wear resistance in zirconia enhances the safety and effectiveness of medical implants, leading to better patient outcomes.
Wear Resistance Benefits:
- Extended Lifespan: Higher wear resistance contributes to longer implant durability.
- Reduced Costs: Lower maintenance and replacement rates lead to cost savings.
- Increased Patient Satisfaction: Enhanced performance reduces the likelihood of complications.
| Wear Resistance Metrics | Importance | Target Value |
|---|---|---|
| Wear Rate | Measurement of material loss over time | < 0.5 mm/year |
| Surface Hardness | Resistance to surface deformation | > 1200 HV |
| Friction Coefficient | Determines interaction with biological surfaces | < 0.5 |
Why Are Surface Properties Important for Zirconia Powder?
Surface properties of zirconia powder play a significant role in its performance as an implant material. These properties can directly influence the material’s adhesion to surrounding tissues, affecting overall biocompatibility and integration.
One key aspect of surface properties is roughness. A properly optimized roughness can enhance osseointegration, allowing the bone to grow into the implant securely. This connection is vital for stable and long-lasting implants.
Another important property is surface energy, which affects how fluids and tissues interact with the zirconia. Higher surface energy can be beneficial as it may promote better biological responses.
Coatings can also alter surface properties, improving aspects like wear resistance or biocompatibility. For instance, applying a bioactive coating can encourage bone growth around the implant, ensuring better functionality.
Understanding surface properties is crucial for manufacturers and healthcare providers to optimize zirconia for specific applications, ultimately enhancing patient safety and device effectiveness.
| Surface Property | Impact | Optimal Range |
|---|---|---|
| Roughness | Affects tissue adhesion | 1-2 μm |
| Energy | Determines interaction with biological systems | > 50 mJ/m² |
| Coating Type | Alters wear/bioactivity characteristics | Varies by application |
Which Regulatory Standards Govern Zirconia Use in Implants?
The use of zirconia powder in medical implants is governed by several regulatory standards that ensure safety and efficacy. Regulatory bodies, such as the FDA and ISO, set guidelines for materials used in medical devices.
The ISO 10993 series is fundamental, detailing the biological evaluation of medical devices. Specific standards within this series outline testing requirements for biocompatibility, ensuring that zirconia can be safely used in the human body without adverse effects.
Furthermore, adherence to Good Manufacturing Practices (GMP) is crucial for manufacturers. These practices emphasize the importance of quality control and consistency in production, ensuring that zirconia meets the required specifications.
By following these regulations, manufacturers can not only achieve compliance but also instill confidence in their products among healthcare professionals and patients alike.
Key Regulatory Considerations:
- Biocompatibility Testing Standards: Ensure materials are safe for bodily use.
- Quality Control Protocols: Maintain product consistency and reliability.
- Documentation and Approval Processes: Required for market entry and trust.
| Regulatory Standard | Focus Area | Key Requirement |
|---|---|---|
| ISO 10993-1 | Biocompatibility evaluation | Comprehensive biological testing |
| FDA Guidance | Premarket approval for medical devices | Submission of safety data |
| ISO 13485 | Quality management systems | Implementation of GMP practices |
How Can Manufacturers Ensure Quality in Zirconia Production?
Manufacturers must take several steps to guarantee the quality of zirconia powder used for medical applications. The production process must start with high-quality raw materials. This sets the foundation for consistent and reliable performance.
Quality control during processing is essential. Regular testing of properties such as particle size distribution, per the ISO guidelines, helps ensure compliance with specifications. Employing advanced techniques, like X-ray diffraction, can confirm the phase purity of the material.
Supplier evaluations and audits are also critical. Establishing strong relationships with reliable suppliers ensures that materials consistently meet quality standards. Collaboration can lead to shared insights and improvements in production processes.
Ultimately, investing in quality assurance not only boosts the performance of zirconia but also builds trust with customers, healthcare professionals, and regulatory bodies.
| Quality Assurance Steps | Description | Outcome |
|---|---|---|
| Raw Material Selection | Choosing high-purity zirconia | Consistent performance |
| In-Process Testing | Regular monitoring during production | Compliance with specifications |
| Supplier Audits | Evaluating suppliers for quality | Reliable material sourcing |
What Innovations Are Shaping Zirconia Powder Applications?
The field of zirconia powder for medical implants is continually evolving, driven by ongoing research and technological advancements. One significant area of innovation is how zirconia’s composition is modified to enhance its properties.
Adding dopants, such as yttria, can stabilize the zirconia structure. This stabilizes it against phase transformations that can occur under stress, leading to increased durability.
Another innovative approach involves developing composite materials where zirconia is combined with bioactive glasses, enhancing osseointegration. This approach opens up new avenues for improving patient outcomes in dental and orthopedic applications.
Understanding these advancements is vital for manufacturers and healthcare professionals looking to leverage the latest technologies to improve implant performance.
| Innovation Area | Description | Impact |
|---|---|---|
| Composition Modification | Using dopants to enhance stability | Increased durability |
| Composites | Integrating bioactive materials with zirconia | Enhanced osseointegration |
| Surface Treatments | Applying coatings to optimize performance | Improved biocompatibility |
How Do Different Applications Influence Zirconia Requirements?
Zirconia powder is used in various applications, each with unique requirements. For dental implants, aesthetic properties play a significant role, whereas orthopedic implants prioritize mechanical strength.
In dental applications, the color of zirconia is crucial for ensuring a natural appearance in prosthetics. Manufacturers often utilize shaded zirconia to match surrounding teeth.
Conversely, orthopedic implants are subjected to higher loads and require enhanced mechanical properties. Here, the focus is on properties like high flexural strength and wear resistance.
Understanding these application-specific requirements helps manufacturers tailor their products, ensuring that zirconia meets the needs of various medical scenarios effectively.
| Application Type | Key Requirements | Focus Area |
|---|---|---|
| Dental Implants | Aesthetic appearance | Color matching |
| Orthopedic Implants | Mechanical properties | Strength and durability |
| Surgical Tools | Precision and sharpness | Hardness |
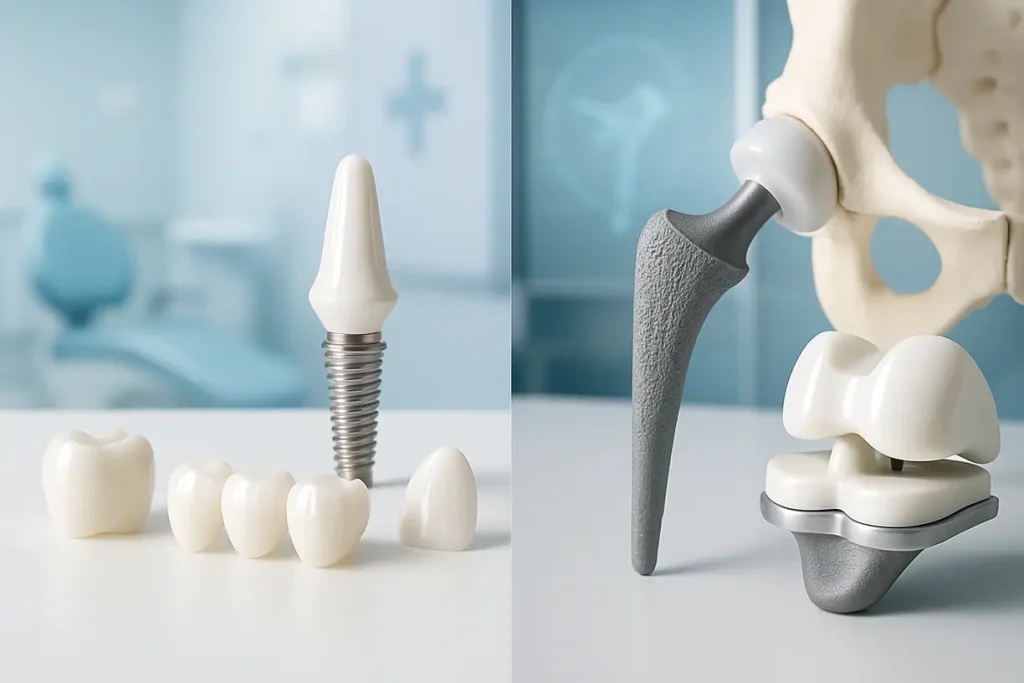
This image depicts zirconia applications in dental and orthopedic implants, showcasing its versatility in medical usage.
What Are the Costs Associated with Zirconia Powder in Medical Use?
When considering zirconia powder for medical applications, understanding the associated costs is crucial. A breakdown of costs can help decision-makers make informed choices.
Production costs are often higher for zirconia compared to other materials, attributable to the advanced techniques and quality standards required. However, the long-term benefits, such as durability and reduced need for replacements, can offset these initial expenses.
In addition to production, logistics and regulatory compliance costs also impact overall expenses. It is vital for organizations to account for these factors when evaluating the total cost of ownership for zirconia implants.
Ultimately, while the initial investment may be significant, the long-term value of using high-quality zirconia can lead to better patient outcomes and reduced overall costs.
Cost Considerations for Zirconia Use:
- Initial Production Costs: Investment in quality raw materials and advanced technologies.
- Regulatory Compliance: Costs associated with safety testing and approvals.
- Life Cycle Costs: Long-term benefits outweigh initial expenses.
| Cost Factor | Description | Implication |
|---|---|---|
| Production Costs | Higher costs for quality production | Initial investment required |
| Regulatory Compliance | Expenses related to meeting safety standards | Necessary for market entry |
| Total Cost of Ownership | Combines all costs over the implant’s life | Evaluates long-term value |
Conclusion
This article highlighted the key standards required for zirconia powder in medical implants, including biocompatibility, mechanical stability, and wear resistance. Understanding these factors equips decision-makers with the knowledge needed to choose the right materials for their applications. A clear benefit of focusing on these standards is improved patient satisfaction and outcomes, translating to greater trust in healthcare solutions. At Global Industry, we are committed to providing expertise and resources to help your business make informed decisions. To learn more about how we can support your material selection and improve your product offerings, reach out to us today.
FAQ Section
Q1: What makes zirconia powder suitable for medical implants?
Zirconia powder is highly regarded for its exceptional biocompatibility, meaning it can safely integrate with human tissues without causing adverse reactions. Additionally, its mechanical strength allows it to withstand significant loads and stresses, making it ideal for weight-bearing implants. Moreover, zirconia’s wear resistance reduces the risk of implant degradation over time, ensuring longevity and reliability in various medical applications, including dental and orthopedic devices.
Q2: How do biocompatibility standards impact patient safety?
Biocompatibility standards are critical in the development of medical implants because they ensure that materials do not elicit harmful responses from the body, such as inflammation or allergic reactions. These standards are established by organizations like ISO and FDA, which require extensive testing to confirm that materials interact positively with biological tissues. Adhering to these standards minimizes the risk of complications post-implantation, ultimately safeguarding patient health and enhancing recovery outcomes.
Q3: What tests are performed to assess zirconia’s mechanical properties?
Several mechanical property tests are essential for evaluating zirconia’s performance in medical applications. Key assessments include flexural strength tests, which measure the material’s resistance to bending forces, and fracture toughness tests, which evaluate its ability to resist crack propagation under stress. Additional tests, such as compressive strength evaluations, help determine zirconia’s effectiveness in supporting heavy loads. These tests ensure that zirconia meets the rigorous demands of various medical implants.
Q4: How can manufacturers verify compliance with zirconia powder standards?
Manufacturers can verify compliance with zirconia powder standards through a systematic approach that includes rigorous testing and quality assurance measures. This process often involves conducting standardized tests as outlined by regulatory bodies like ISO and FDA. Additionally, manufacturers may undergo audits by third-party organizations to ensure their processes adhere to Good Manufacturing Practices (GMP) and other relevant quality standards. Documentation of these processes is vital for demonstrating compliance and fostering trust among stakeholders.
Q5: What cost factors should be considered when sourcing zirconia powder?
When sourcing zirconia powder, several cost factors must be considered to evaluate overall economic feasibility. Initial production costs are often higher for zirconia due to the need for high-quality raw materials and advanced processing techniques. Regulatory compliance costs, including testing and certification expenses, can also add to the total expenditure. Additionally, manufacturers should account for long-term costs related to durability and reduced replacement needs, as high-quality zirconia can lead to fewer complications and ultimately lower lifetime costs for healthcare providers.