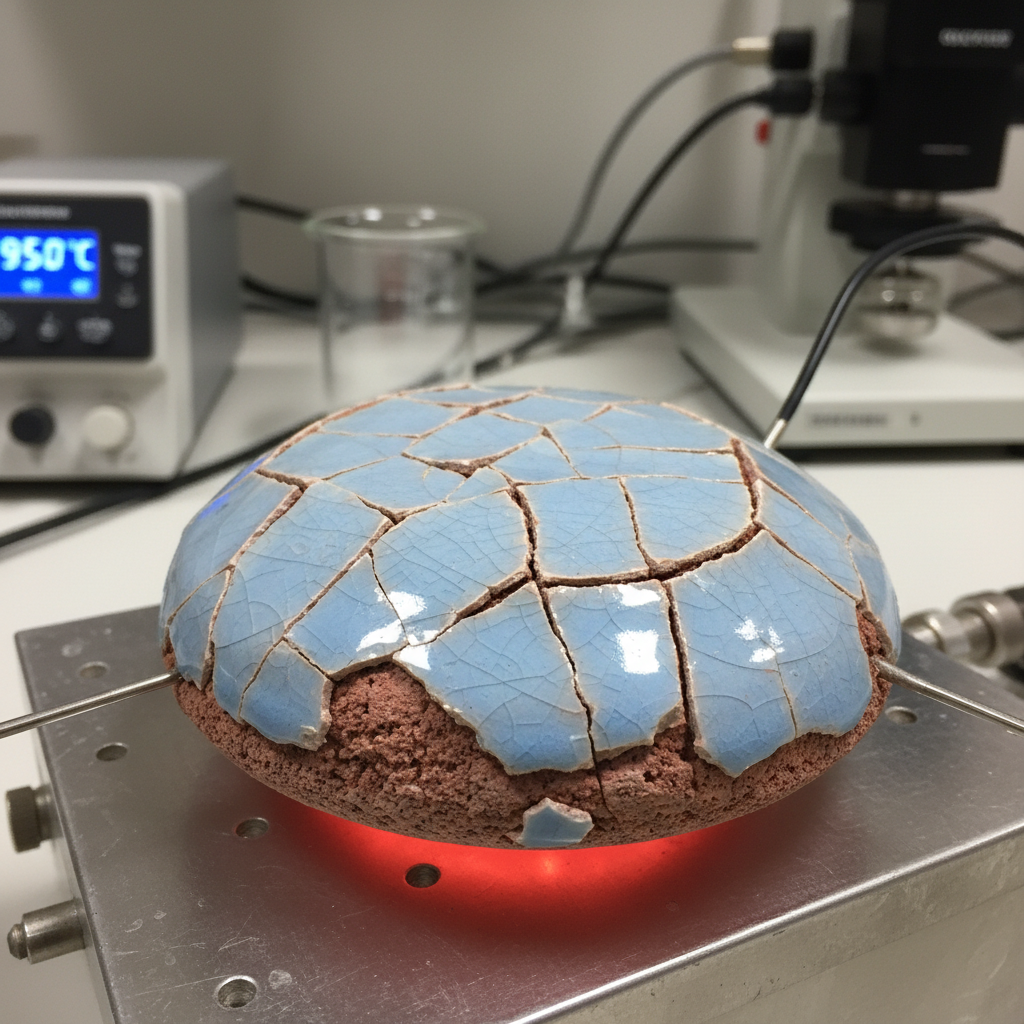
You open a kiln expecting perfection. Instead, you hear a distinct ping of crazing glaze echoing from the shelf. That sound represents a fundamental breakdown in materials science known as thermal expansion mismatch. This invisible force destroys yield, ruins reputation, and burns profits by creating structural defects you simply cannot sell. Here is the deal: ignoring the science of expansion guarantees failure, but mastering it ensures industrial-grade durability. By understanding how materials expand and contract, you can transform your production from a game of chance into a precise engineering discipline.

What Defines High Ceramic Quality?
High ceramic quality is not merely an aesthetic judgment regarding surface gloss or color. It is a measurable state of structural equilibrium defined by a defect-free internal matrix and superior mechanical durability. When assessing quality, professional engineers look for the total absence of crazing, shivering, or dunting. These defects signal an internal war between materials. True quality means an object behaves as a singular entity rather than two separate layers fighting for space. A high-quality piece must withstand thousands of dishwasher cycles, rapid microwave heating, and physical impact without catastrophic failure.
You might be wondering, what constitutes true durability? It is the structural integrity of the interface layer found between a clay body and glaze. If an internal matrix cannot handle temperature fluctuations, a product is fundamentally flawed regardless of how beautiful it appears on a shelf. Industrial applications demand high resistance to thermal shock and impermeability to liquids. A porous ceramic piece acts like a rigid sponge. It absorbs fluids through microscopic breaches. This absorption leads to bacterial growth and hygiene failure. Therefore, quality relies on stress-managed integration.
Key Quality Metrics
| Metric | Standard Requirement | Technical Implication | |
|---|---|---|---|
| Tensile Strength | > 30 MPa | Prevents snapping under physical load | |
| Water Absorption | < 0.5% (Vitreous) | Ensures hygiene and prevents moisture expansion | |
| Glaze Compression | Moderate | Stops delayed crazing and increases strength | |
| Thermal Shock | > 150°C Delta T | Guarantees survival during rapid temperature changes | |
| Mohs Hardness | > 5.0 | Resists cutlery marking and abrasion |
How Does Thermal Expansion Impact Ceramic Quality?
Thermal expansion impacts ceramic quality by dictating a precise rate at which a clay body and glaze change size during heating. Every material possesses a Coefficient of Thermal Expansion (CTE). If these rates are not synchronized, resulting mismatch creates destructive mechanical forces. These forces tear work apart from the inside out. Stress generates when two bonded materials attempt to move at different speeds. Kinetic energy increases as heat rises. Atoms vibrate more vigorously while bond lengths stretch. This causes macroscopic expansion across an entire vessel.
This is where it gets interesting: glaze is essentially glass while a body is a crystalline matrix. They naturally behave differently under heat. Glass generally expands in a smooth linear fashion. A clay body containing quartz expands with sudden jumps in volume. Reconciling these two behaviors is a primary challenge for engineers. If a body shrinks significantly more than its glaze, it crushes that coating. If a glaze shrinks more than its body, it stretches until it snaps. Mismatched expansion rates guarantee structural failure. The magnitude of failure depends entirely on the size of the CTE gap between body and surface.
Material Expansion Behaviors
| Material Phase | Expansion Characteristic | Risk Level | |
|---|---|---|---|
| Amorphous Glass | Linear / Predictable | Low risk if chemistry is balanced | |
| Alpha Quartz | Sudden jump at 573°C | Severe cracking (Dunting) | |
| Cristobalite | Sudden jump at 220°C | High risk of “delayed dunting” | |
| Feldspar | Moderate / Steady | Acts as buffer to smooth curves |
Why Does Glaze Fit Alter Ceramic Quality?
Glaze fit alters ceramic quality by determining whether a surface remains an impermeable protective layer or becomes a network of fractures. A proper fit uses physics to clamp a glaze onto ware like a steel hoop holds a wooden barrel together. Engineers do not aim for a perfect match. They aim for a beneficial mismatch. A tight fit means glaze has a slightly lower CTE than a clay body. As a pot cools, clay contracts more than glass. This forces a body to squeeze glaze tight.
But here is the kicker: ceramics are incredibly strong under compression but notoriously weak under tension. Placing glaze in tension guarantees failure. This is crazing. It manifests as a web of fine cracks. While sometimes considered artistic, crazing in functional ware is a hygiene defect. Conversely, if fit is too tight, stored energy seeks release. It pops glaze off edges in razor-sharp flakes. This is shivering. Shivering is a massive liability as glass shards can enter food. Controlled compression is a non-negotiable requirement for quality. It closes microscopic fissures while increasing effective strength.
Stress States and Consequences
| Fit Condition | Stress State | Physical Phenomenon | Consequence | |
|---|---|---|---|---|
| Crazing | Tension | Glaze is stretched until fracture | Leaking / Bacterial Harboring | |
| Shivering | High Compression | Glaze is squeezed until buckled | Sharp peeling / Injury Risk | |
| True Fit | Mild Compression | Glaze is tightly clamped | Increased Strength / Durability | |
| Delayed Crazing | Moisture Expansion | Glaze cracks months later | Long-term product failure |
When Does Silica Inversion Risk Ceramic Quality?
Silica inversion risks ceramic quality when a firing schedule ignores rapid volume changes of quartz molecules. This is not a chemical reaction. It is a physical phase change. At exactly 573°C, Alpha quartz molecules rearrange into Beta quartz. This transition involves a volume increase of roughly 2% during heating. It involves a corresponding contraction during cooling. While 2% sounds small, it is massive in rigid ceramics. It provides enough force to snap a heavy sculpture in half instantly.
Think about this: rushing through this temperature zone is a primary cause of dunting. Dunting cracks are sharp and clean. They often travel through both body and glaze. If cooling is too rapid through 573°C, thermal gradients create shear stress. One part of a pot has shrunk while another remains expanded. This size difference rips ware apart. Cristobalite undergoes a similar but more violent inversion around 220°C. High-fire stoneware often contains significant cristobalite. Rapid cooling at the very end of a cycle can shatter ware that survived quartz inversion. You must slow down firing through these specific zones to preserve quality.
Silica Phase Transitions
| Mineral Phase | Inversion Temp | Volume Change | Practical Strategy | |
|---|---|---|---|---|
| Alpha/Beta Quartz | 573°C (1063°F) | ~2.0% (Sudden) | Slow ramp (<50°C/hr) | |
| Cristobalite | 220°C (428°F) | ~3.0% (Very Sudden) | Gentle cooling below 250°C | |
| Tridymite | 117°C – 163°C | Moderate | Monitor low-temp cooling |
How Does Firing Speed Affect Ceramic Quality?
Firing speed affects ceramic quality by creating thermal gradients within ware. Ceramics are excellent insulators. Heat penetrates them slowly. A fast firing ramp ensures temperature on a thermocouple differs significantly from temperature inside a clay wall. When a kiln heats too fast, an outer surface expands before a core does. Layers of clay slide against each other microscopically. This creates shear stress. While a piece may not break visibly, it develops a network of micro-fissures. These weaken structural integrity.
Here is the truth: thicker ware requires exponentially slower firing speeds. A one-inch thick sculpture may require a cycle four times longer than a thin mug. Fast firing also seals a surface before carbon and sulfur gases escape. This causes pinholes or bloating. Patience in firing directly improves yield. The cost of electricity for an extended firing is always lower than the cost of a ruined kiln load. You must tailor your schedule to the thickest piece in a load. Uniform heat distribution is essential for consistent thermal expansion behavior across an entire batch.

Firing Ramp Optimization
| Firing Stage | Risk Factor | Optimal Strategy | Reason | |
|---|---|---|---|---|
| 0-200°C | Steam Explosion | Candling Hold | Evaporates pore water safely | |
| 500-650°C | Quartz Inversion | Slow Ramp | Prevents dunting from volume shock | |
| Peak Temp | Uneven Heat Work | 20 Minute Soak | Equalizes temperature in kiln | |
| Cooling | Thermal Shock | Natural Cool | Prevents rapid contraction stress |
Why Do Additives Change Ceramic Quality?
Additives change ceramic quality by chemically altering thermal expansion behavior of glaze without necessarily changing visual appearance. This is the domain of limit formulas and oxide chemistry. Every oxide in a recipe contributes to total expansion. High expansion oxides like Sodium and Potassium make glazes melt at low temperatures but cause crazing. Stabilizers like Alumina and Silica generally lower expansion. They add hardness. Engineers use these materials as chemical levers.
Ready for the best part? Zirconium Silicate is often called magic dust for ceramic engineering. It has an extremely low CTE. Adding just 5% to 10% Zircon to a high-expansion glaze acts like adding steel rebar to concrete. It drastically stabilizes a matrix. It drops expansion enough to stop crazing completely. Strategic use of additives allows you to fine-tune glaze fit. You can correct defects at a molecular level. This avoids overhauling an entire base recipe. It provides a precise method for engineering durability into aesthetic finishes.

Common Additive Effects
| Additive/Oxide | Thermal Effect | Visual Effect | |
|---|---|---|---|
| Zircon (ZrSiO4) | Drastically Lowers | Opacifier (Whitens) / Fixes Crazing | |
| Silica (SiO2) | Lowers | Increases Hardness / Raises Melt Temp | |
| Sodium (Na2O) | Drastically Increases | High Gloss / Fluid Melt | |
| Lithium (Li2O) | Lowers | Powerful Flux / Active Melt |
Where Does Porosity Lower Ceramic Quality?
Porosity lowers ceramic quality by allowing absorption of water and biological contaminants. A porous piece acts like a hard sponge. If a clay body is not vitrified, it will absorb liquids through any breach in glaze. This leads to immediate hygiene failure. Bacteria traps within a clay matrix cannot be washed out. Absorbed water expands when heated in a microwave. This causes a pot to crack from inside out. Porosity is disastrous for functional ware used in dining or sanitary applications.
What’s the real story? Porous ceramics suffer from a defect known as moisture expansion. Clay bodies absorb humidity from atmosphere over weeks or months. Water molecules wedge themselves into clay minerals. A clay body physically expands while its glaze does not. This stretches glaze until it snaps. This delayed crazing often appears months after sale. It damages brand reputation significantly. Zero porosity through full vitrification defines the highest standard of quality. It ensures ware remains sanitary and mechanically stable over a lifetime of use.

Porosity Standards by Type
| Ware Type | Target Porosity | Quality Standard | |
|---|---|---|---|
| Earthenware | 10-15% | Low (Must be glazed perfectly) | |
| Stoneware | 1-3% | High (Functional / Durable) | |
| Porcelain | < 0.5% | Premium (Vitreous / Translucent) | |
| Bone China | < 0.3% | Superior Strength / Whiteness |
How Does Cooling Rate Determine Ceramic Quality?
Cooling rate determines ceramic quality by locking in crystal structure. While firing builds ceramic, cooling defines its final stress state. A rushed cooling cycle undoes chemical work performed during heating. Glazes have an annealing range similar to glass. This is a zone where liquid transitions to solid. Rapid cooling freezes glass structure in a chaotic state. It traps high internal tension. Slow cooling allows molecules to align. It relaxes internal stress.
It gets better: controlled cooling can actually enhance surface aesthetics. Holding a kiln at specific temperatures during a drop allows glazes to stay fluid longer. This heals micro-bubbles known as pinholes. It smooths over surface imperfections. It creates a tougher surface. Patience during cooling is a final safeguard. Crashing a kiln by opening it at high temperatures shocks materials. It induces invisible fatigue. This fatigue leads to early failure under minor stress. You must respect physics of contraction as much as expansion.

Cooling Phase Management
| Cooling Phase | Action | Quality Impact | |
|---|---|---|---|
| Peak to 1000°C | Moderate Cool | Crystal development / Surface smoothing | |
| 573°C (Quartz) | Slow / Hold | Prevents body cracks (Dunting) | |
| 200°C to Room | Natural Cool | Prevents thermal shock from drafts | |
| Annealing Zone | Controlled Soak | Relieves internal material stress |
Why Does Clay Composition Set Ceramic Quality?
Clay composition sets ceramic quality by establishing a baseline Coefficient of Thermal Expansion. You cannot force a low-expansion glaze to fit a high-expansion body if chemistry is fundamentally incompatible. Clay is a canvas. If a canvas shrinks unpredictably, paint will crack. Silica is a primary glass-former. It dictates expansion rates more than any other material. Free silica acts as filler but contributes heavily to inversion risks. Chemically combined silica locks into mullite structures which are stable.
Bottom line: precise formulation of a clay body is an immovable foundation. High-quality ceramics start with a clay recipe engineered for a specific firing range. If you change a clay supplier, you effectively change physics of your product line. Adjusting silica mesh size in a body can radically change resistance to thermal shock. Finer silica reacts more completely to form glass. Coarser silica remains as quartz particles acting as aggregate. Successful production relies on a consistent clay recipe providing a known target for glazes.

Clay Body Components
| Component | Function | Impact on CTE | |
|---|---|---|---|
| Silica Sand | Structure / Glass Former | Increases significantly | |
| Kaolin | Plasticity / Alumina Source | Neutral / Stabilizing | |
| Ball Clay | Plasticity / Free Silica | Increases moderately | |
| Feldspar | Flux (Melter) | Variable / Controls vitrification |
How Does Measurement Ensure Ceramic Quality?
Measurement ensures ceramic quality by replacing guesswork with scientific data. In a professional setting, you cannot rely on the sound of a ping to detect failure. You need quantifiable metrics. Dilatometry is a gold standard for testing ceramic interactions. A dilatometer heats a small sample of ceramic. It measures physical expansion with micrometer precision. It plots a graph of expansion versus temperature. This visualizes exactly where materials diverge. It identifies inversion points clearly.
This is where it gets interesting: a dilatometer graph reveals a precise mismatch gap between body and glaze. You can literally see if a glaze is under tension or compression. This allows you to adjust recipes by fractions of a percent. Autoclaves are also used to test for delayed crazing. Ceramics are subjected to high-pressure steam. This simulates years of aging in a few hours. If a glaze crazes after autoclaving, a batch is rejected. Scientific measurement is the only way to guarantee consistent industrial-grade quality.

Professional Testing Methods
| Test Method | Purpose | Equipment Needed | |
|---|---|---|---|
| Dilatometry | Measure CTE / Exact Fit | Dilatometer | |
| Autoclave | Test Moisture Expansion | High-pressure Steam Vessel | |
| M.O.R. | Test Structural Strength | Hydraulic Stress Tester | |
| Steger Test | Measure Glaze Stress | Bending Bar Apparatus |
Conclusion
You have seen how thermal expansion mismatch destroys hard work through crazing, shivering, and structural failure. These defects are not mysteries. They are predictable outcomes of physics. By optimizing firing schedules to manage silica inversion and using additives like Zircon to tune glaze fit, you can transform yield. Do not accept failure as part of a process. Embrace measurement and engineering. Master the science of expansion to guarantee that every piece leaving your kiln is durable, safe, and flawless.
FAQ Section
Q1: What is the main cause of crazing? Crazing occurs when a glaze shrinks more than a clay body during cooling. This places the glaze under tension until it fractures to relieve stress.
Q2: How does silica affect thermal expansion? Silica has a high coefficient of thermal expansion. Adding free silica to a clay body generally increases the body’s expansion rate which can help fit low-expansion glazes.
Q3: What is the difference between crazing and shivering? Crazing is a network of cracks caused by tension forces. Shivering is the peeling of glaze off edges because it is under excessive compression forces.
Q4: How do I test for thermal shock resistance? You can test thermal shock resistance by heating a ceramic piece in an oven to a specific temperature and immediately plunging it into ice water to see if it cracks.
Q5: Why is the quartz inversion point important? At 573°C quartz changes shape and volume suddenly. Firing too fast through this temperature causes immediate structural cracking known as dunting.